Background.As a T cell driven process where release of inflammatory cytokines as a result of the proliferation and on target cell kill by chimeric antigen receptor (CAR)-T cells, cytokine release syndrome (CRS) can be potentially targeted by adoptive therapy with T regulatory (Treg) cells. Specifically, allogeneic cord blood (CB) derived Treg cells have now shown safety and efficacy in graft vs host disease (GVHD), we hypothesized that CB Treg cell therapy can be exploited for treatment of CRS.
Method.Xenogenic lymphoma model was created using NSG mice where 0.3x106 GFP-labeled Raji cells were injected on day 0 in all mice followed by 0.3x106 cells of i) mock-CAR T, ii) no CART, ii) CD19-CAR T cells on day +5. Additional injections of 1x107 CB Treg cells on day +11, +18, +25 were added to the no CAR T arm and the CD19-CAR T arm such that there were 3 mice per arm. Mice were followed for weight, GVHD score and survival. Non-invasive bioluminescence was used to perform serial imaging to evaluate the tumor burden. Serial blood was drawn for cell analysis and cytokine assay.
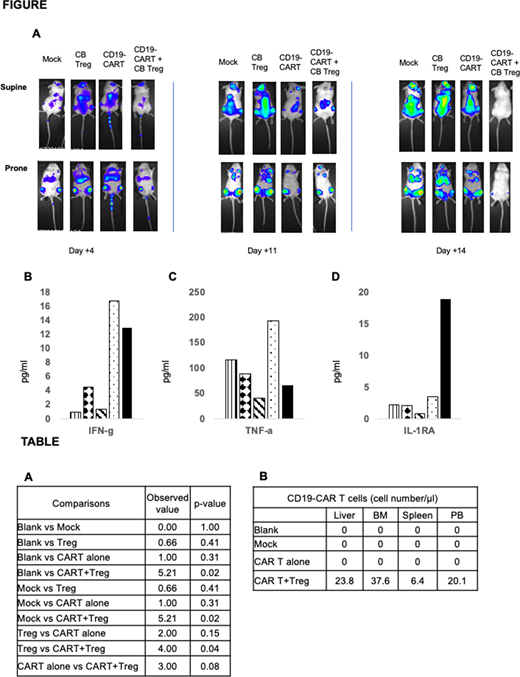
Result. As shown in figure A, in vivo proliferation of GFP-labeled Raji cells was evident in all mice day by day +4. CD19-CAR T but not the mock-CAR T cells decreased the tumor burden at day+11. However, at day +14 all mice including CD19-CAR T cell recipients showed progression whereas CD19-CAR T+CB Treg cell recipient showed no evidence of bioluminescence. A superior survival in the CD19-CAR T+CB Treg cells recipients was evident when compared to other treatment arms (Table A). At the time of euthanasia, different organs were evaluated for the detection of the CD19-CART cells and were recovered only in the CD19-CART+CB Treg cells recipients (Table B) . The CD19-CAR T recipients showed an increase in the inflammatory cytokines on day +16 PB samples including IFN-gamma (Figure B) and TNF-alpha (Figure C) which were decreased in the CD19-CAR T + CB Treg arm. Furthermore, a reciprocal increase of the anti-inflammatory cytokine IL-1RA was observed in the CD19-CAR T + CB Treg arm compared to the CD19-CAR T alone (Figure D).
Conclusion. The addition of CB Treg cells to CD19-CAR T cells in a xenogenic lymphoma model led to dampening of the cytokine storm and improved on target efficacy of CAR T cells. This combination should be examined in clinical setting.
Sadeghi:Cellenkos Inc.:Current Employment.Nastoupil:Genentech, Inc.:Honoraria, Research Funding;Karus Therapeutics:Research Funding;Bayer:Honoraria;Gamida Cell:Honoraria;Gilead/KITE:Honoraria;Novartis:Honoraria, Research Funding;Merck:Research Funding;TG Therapeutics:Honoraria, Research Funding;LAM Therapeutics:Research Funding;Janssen:Honoraria, Research Funding;Pfizer:Honoraria, Research Funding;Celgene:Honoraria, Research Funding.Patel:Oncopeptides:Consultancy;Janssen:Consultancy, Research Funding;Precision Biosciences:Research Funding;Takeda:Consultancy, Research Funding;Nektar:Consultancy, Research Funding;Celgene:Consultancy, Research Funding;Bristol Myers Squibb:Consultancy, Research Funding;Poseida:Research Funding;Cellectis:Research Funding.Parmar:Cellenkos Inc.:Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.